
Hello
I'm David Vidmar
Data Scientist, Physics PhD
-
- dvidmar.dev@gmail.com
-
- Click here for my LinkedIn page
I work as a data scientist at 4th-IR, where I work closely with clients to understand their professional needs and develop machine learning and AI tools to address them. As a former physicist, my passion involves employing the quantitative rigor of physics to solve complex real-world problems. I've applied this passion throughout my career by designing and implementing novel means of analyzing data.
Develop machine learning tools with applications across various industries. I work collaboratively as part of a team of data scientists and software engineers.
Conducted research as a graduate student designing methods of detecting arrhythmia sources from clinical data, as well as using stochastic methods to infer important statistics of fibrillation. See "PhD Projects" for more information.
Participated in a summer internship working on RhythmView software for mapping electrical activity in the heart.
Conducted research as an undergraduate student, analyzing birdsong data using methods in nonlinear time series analysis and chaos detection in noisy systems.
Obtained Ph.D. after presenting my dissertation to a committee of experts from physics and math.
Obtained M.Sc. in physics after passing qualifying exams and required coursework.
Obtained B.Sc. in physics, graduating with honors.
I worked as a teaching assistant in the physics department for four years, where I taught a wide array of topics in both lecture and laboratory courses. In 2015 I was chosen to design and implement the first set of video lectures using "Learning Glass" technology for a theoretical mechanics course taken by all science and engineering undergraduates. These videos are now given to students as a complement to the course each semester it is taught. Below I have included select quotes from my teaching evaluation for my most recent course, as well as a sample clip from one of my video lectures.
During a normal human heartbeat, an electrical wave of excitation spreads out evenly across cardiac tissue. This electrical signal causes contraction of the heart muscle, resulting in the pumping of blood throughout the body. If this electrical wave becomes mangled, the resulting contraction can become substantially altered, leading to an arrhythmia. The most serious type of arrhythmia, known as fibrillation, arises when the heart's contraction becomes entirely uncoordinated and ineffective, with its motion reduced to chaotic quivering. My PhD research focused on understanding the complex dynamical state underlying human fibrillation by exploring the relationship between theoretical principles, computational simulations, and clinical data.

Images by wikipedia user JHeuser, distributed under CC BY-SA 3.0
Though fibrillation in humans can exist indefinitely, it is most commonly seen in episodes which last from minutes to days, eventually terminating back to normal sinus rhythm. An obvious question arises: what is the mechanism allowing for the chaos inherent to fibrillation to persist for extended periods of time? Put another way, what exactly is perpuating this arrhythmia and how can we stop it?
Recent findings point to the importance of consistent rotational patterns of conduction as playing an important role in the maintanence of this arrhythmia. These rotational patterns are grounded in extensive theoretical and computational studies of the heart and are hypothesized to act as the drivers of disorganization. A sample simulation of this hypothesis is shown to the right, where a central rotational pattern exists with wavefronts breaking up far from the core.
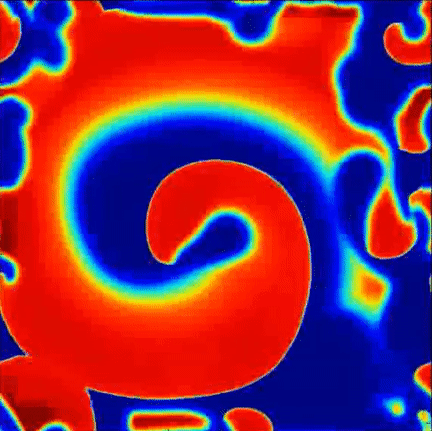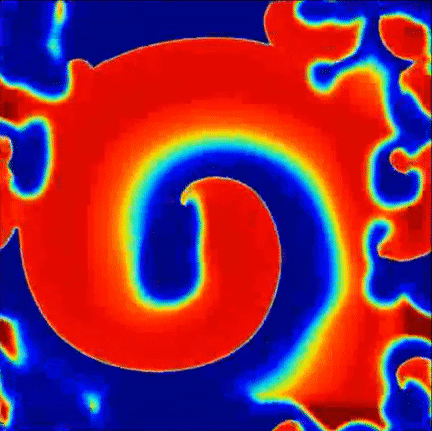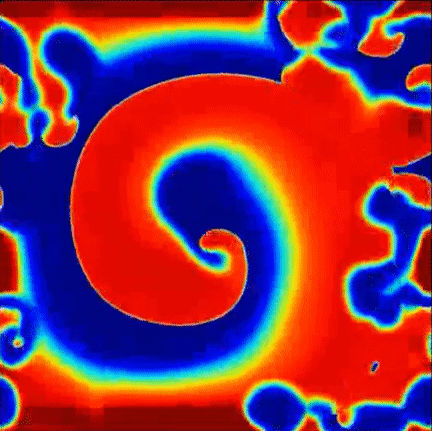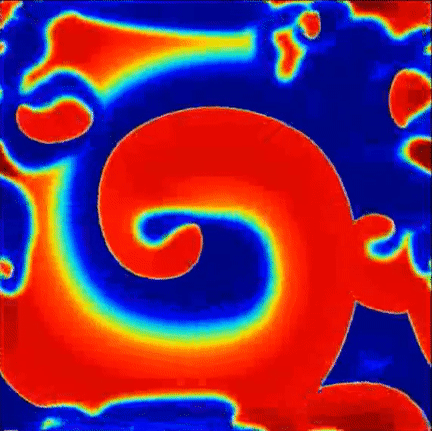
Simulation of rotational pattern surrounded by breakup, red is excited tissue and blue is resting tissue.
To better understand the role that various conduction patterns play in human fibrillation, it becomes necessary to collect and analyze clinical data from patients. This is achieved through use of a basket catheter, which records electrical signals at various points on the surface of the heart during an episode of arrhythmia. These signals are then processed and used to create patient-specific videos of the underyling electrical activity.
One focus of my work was to design and implement a methodology to make the interpretation of these complex activation videos easier for physicians and scientists, as well as providing quantiative measures of any conduction patterns present. To do so, we designed a novel algorithm which accurately identifies and visualizes the vector flow field of electrical activity in these videos. This is outlined in our publiction "Determing Conduction Patterns on a Sparse Electrode Grid" (see below) as well as in our patent application WO2017165846A1. With this tool we can conduct systematic studies on the dynamical evolution of arrhythmia episodes.
Activation video during clinical fibrillation, with the computed flow field in green and activation in white. Reproduced from Vidmar et al (2016), Physical Review E. Copyright APS.
Vidmar, D., Narayan, S. M., Krummen, D.E., Rappel, W.-J. Phys. Rev. E - Rapid Communication (2016)
Vidmar, D., Krummen, D.E., Hayase, J., Narayan, S. M., Ho, G., Rappel, W.-J. JACC: Clinical Electrophysiology (2017)
Vidmar, D., Narayan, S. M., Rappel, W.-J. Am. J. Physiol. Heart (2015).
Vidmar, D., Alhusseini, M., Narayan, S. M., Rappel, W.-J. Frontiers in Physiology (2018)
Alhusseini, M. & Vidmar, D., et al. J. Cardiovasc. Electrophysiol. (2017)
Narayan, S. M., Zaman, J., Vidmar, D., Wang,P. , Rappel, W.-J. Cardiac Electrophysiology: From Cell to Bedside, 7e (2017).